Articles
Minimizing Assays- Increasing Returns
Wed, 09/20/2006 - 10:48am
Researchers utilizing HTS screening methods have been charged with finding greater active compound hits in shorter periods of time. More often than not, costs for screening methods had increased as throughput increased. In order to manage their goals within cost boundaries, HTS researchers have turned to manufacturers of HTS-related products and equipment to help them generate more efficiencies.
Screening assays for therapeutic compounds were at one time performed in glass vials or tubes - an arduous and expensive task, generating very little information over a long period of time. This method was revolutionized in the 1970's with the introduction of 96-well microplates specific for immunoassays. Researchers quickly modified screening assays into this new format; testing more compounds in less time, and with significantly lower raw material costs. Initial costs to validate the new assay format were quickly recouped through the overall reduction in raw materials.
Within two short decades, the screening industry pushed the limits of technology further with the introduction of higher density microplates in 384-well and 1536-well formats. In each configuration, more compounds could be screened with less raw material costs - a significant increase in "bang" using fewer "bucks". Although higher density microplates are being developed, Gary Barush, Director of Marketing and Sales at BioTek Instruments, Inc., questions whether this assay development trend will continue beyond 1536-well formats.
"Issues that are present but manageable in 96-well and 384-well microplates can develop as daunting problems in ultra-high density microplates," he noted. "Evaporation and background interference are just a few obstacles that can pose major problems in this format. At this point, assay optimization in compound screening is at a plateau until we can develop completely new modes of chemistry and technology."
Additionally, Barush states that any savings from moving to ultra-high density microplates is easily countered by the cost of the microplates as well as the microplate equipment used within the process. The result would be an overall increase, not a decrease, in cost.
In fact, over 95% of microplate users plan to stay in 96-well, 384-well or 1536-well formats in the coming year, and have no plans to transition into higher density microplates for HTS applications.
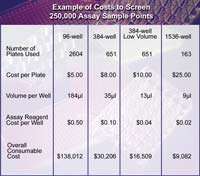
So if ultra-high density microplates aren't the answer, how do researchers find additional cost savings? The answer lies in the reagent volumes. Reagent costs are the most significant recurring expense for HTS compound screening assays and their development. A simple reduction in the reagent volume can add up to a significant reduction in overall expenses. A 384-well low volume microplate, designed specifically for sub-microliter assays, can provide up to a four-fold reduction in working volume when compared to a traditional 384-well microplate.
With the increasing popularity of 384-well low volume microplates, HTS researchers can benefit from reagent cost reduction while operating on the same equipment. The use of sub-microliter assays, using the same overall microplate footprint with a lower reagent volume, is forecast to grow over 60% within the next year as more laboratories search for more results at less overall cost.
Traditionally, liquid dispensers used in this process fell into two categories. Common microliter dispensers are economical for most laboratories, but suffer from decreased accuracy and precision at the lowest volume range. On the other hand, highly accurate nanoliter dispensers have been cost-prohibitive.
The NanoQuot sub-microliter microplate dispenser (BioTek Instruments, Inc., Winooski, VT) bridges the gap between these dispensing groups, combining economy and precision. The unit's eight solenoid valves rapidly deliver volumes from 0.1 to 40 μl, with dispensing accuracy and precision of less than 5% at 1 μl. Flexibility in microplate format — 96-well, 384-well, 384-well low volume, and 1536-well microplates — means that researchers can easily transition into lower volume assay configurations.
As in the historical transition from tubes to microplates, initial costs to validate the assay in a low-volume format are quickly recovered through the significant reduction in reagent expenses. Meanwhile, backward compatibility with existing formats and volumes allows for assay validation in low-volumes while still producing results in the existing format. The same reagents can be used for robust assays, and capital expenses are non-existent as the low-volume assays are processed on existing equipment. Finally, one more bonus exists for the lab manager in the form of flat or reduced labor costs associated with these more efficient assays.
 |
Within two short decades, the screening industry pushed the limits of technology further with the introduction of higher density microplates in 384-well and 1536-well formats. In each configuration, more compounds could be screened with less raw material costs - a significant increase in "bang" using fewer "bucks". Although higher density microplates are being developed, Gary Barush, Director of Marketing and Sales at BioTek Instruments, Inc., questions whether this assay development trend will continue beyond 1536-well formats.
"Issues that are present but manageable in 96-well and 384-well microplates can develop as daunting problems in ultra-high density microplates," he noted. "Evaporation and background interference are just a few obstacles that can pose major problems in this format. At this point, assay optimization in compound screening is at a plateau until we can develop completely new modes of chemistry and technology."
 |
In fact, over 95% of microplate users plan to stay in 96-well, 384-well or 1536-well formats in the coming year, and have no plans to transition into higher density microplates for HTS applications.
So if ultra-high density microplates aren't the answer, how do researchers find additional cost savings? The answer lies in the reagent volumes. Reagent costs are the most significant recurring expense for HTS compound screening assays and their development. A simple reduction in the reagent volume can add up to a significant reduction in overall expenses. A 384-well low volume microplate, designed specifically for sub-microliter assays, can provide up to a four-fold reduction in working volume when compared to a traditional 384-well microplate.
With the increasing popularity of 384-well low volume microplates, HTS researchers can benefit from reagent cost reduction while operating on the same equipment. The use of sub-microliter assays, using the same overall microplate footprint with a lower reagent volume, is forecast to grow over 60% within the next year as more laboratories search for more results at less overall cost.
Traditionally, liquid dispensers used in this process fell into two categories. Common microliter dispensers are economical for most laboratories, but suffer from decreased accuracy and precision at the lowest volume range. On the other hand, highly accurate nanoliter dispensers have been cost-prohibitive.
The NanoQuot sub-microliter microplate dispenser (BioTek Instruments, Inc., Winooski, VT) bridges the gap between these dispensing groups, combining economy and precision. The unit's eight solenoid valves rapidly deliver volumes from 0.1 to 40 μl, with dispensing accuracy and precision of less than 5% at 1 μl. Flexibility in microplate format — 96-well, 384-well, 384-well low volume, and 1536-well microplates — means that researchers can easily transition into lower volume assay configurations.
 |