FDA-approved Stem Cell Trial Dramatically Slows ALS
 For an unheard-of two years, stem cells have slowed the progression of Lou Gehrig’s disease, a devastating condition with a two-to-five year survival rate, in a small group of patients.
For an unheard-of two years, stem cells have slowed the progression of Lou Gehrig’s disease, a devastating condition with a two-to-five year survival rate, in a small group of patients.
“We have now extended the lives of patients with Amyotrophic Lateral Sclerosis (ALS) and significantly improved the quality of their lives. They are now living high-quality lives with this disease,” says Neuralstem CEO Richard Garr.
For 700-800 days, human fetal neural stem cells, cultivated by Neuralstem, have substantially slowed muscle degeneration—the hallmark of ALS—in six patients. There has been “no significant disease progression” in these patients, says American Neurological Association president Eva Feldman. This is rare, says Feldman, who is the study’s principle investigator, and Director of Research of the ALS Clinic at the University of Michigan Health System.
She adds: “Being a Michigan wolverine, I’ll use an analogy. It’s as rare as a red wolf. And that is very rare.”
Before surgery, all six of the extraordinary responders had disease only two years along. All of those also lacked the speaking and swallowing complications of “bulbar ALS.” Nine other patients, eight of whom either had bulbar ALS, or advanced disease of five-plus years, did not respond. So the improvements only appear to occur in patients in an earlier phase of the disease.
But the FDA strictly curtailed the number of stem cells allowed at that stage. Millions more cells will soon be given to new patients.

Feldman was interviewed after presenting the new data on May 17 to researchers at the Romanian Neurological Society Congress. The Phase 1 trial, conducted at Emory University, involved the administration of human fetal spinal cord stem cells to the lower (lumbar) spinal region of nine ALS patients; the upper cervical region of three ALS patients; and both regions to three patients.
In February the trial’s progress persuaded the FDA to greenlight a Phase 2 trial, expected to start this summer. This trial will give 15 new patients millions more cells, and will test for efficacy. The Phase 1 trial, designed simply to test for safety, gave patients only “one tenth the cell doses” the team desires for therapeutic impact, says Garr.
The first major surprise occurred with the trial’s most impressive responder, Atlanta resident Ted Harada, who couldn’t walk without a cane before receiving one million cells, 500,000 on either side of his lower spine. Within months, in March 2011, he had abandoned the cane and participated in a 2.5 mile walkathon, actions considered unprecedented in medical circles. (Notes Feldman: “That’s an understatement.”) In August 2012, Harada became the last of three patients to receive a second dose of cells—this time, to the upper spinal cord.
Harada directed the attention of the general public to the trial. But the medical establishment was already demonstrating “substantial interest,” Feldman says. This was heightened after early results were published in the journal Stem Cells.1
Results continue to hold steady. Says Garr: “No one has ever seen data like this.”
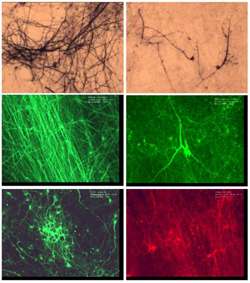
The cells, injected into one or both sides of the lower (lumbar) spinal cord of the patients in the first part of the Phase 1 trial, appear to have survived in the six responding patients long enough to pump out therapeutic doses of neurotrophins, natural chemicals protecting neurons from further degeneration. But autopsies reveal the stem cells also seem to be differentiating into neurons, and hooking up synaptically, as they did in animal models,2 a significant development.
“The cells mature and form synaptic connections on diseased motor neurons in the spinal cords of ALS animal models. It is remarkable actually,” says Feldman. “No small molecule drug has proved effective in man, and neither has any small molecule drug shown the histological and physiological preservation of function that stem cells have in animal models of ALS.”
The manuscript on the final results will be submitted to a journal soon, she says.
In this summer’s Phase 2 trial, Feldman will give millions more cells to 15 more ALS patients. Most will receive the cells in the upper spine, although three will receive cells in both the upper and lower spine. The upper spine is critical. Most ALS patients die when the disease attacks muscles controlling their lungs.
_improved_imag.jpg) Neuralstem’s fetal cells are unlike embryonic stem cells in that they are more differentiated (mature), yet still robust: controllable yet potent. They are unlike fully adult neural stem cells in that they are younger, and possess fewer mutations.
Neuralstem’s fetal cells are unlike embryonic stem cells in that they are more differentiated (mature), yet still robust: controllable yet potent. They are unlike fully adult neural stem cells in that they are younger, and possess fewer mutations.
Neuralstem has many different neural stem cell lines, and has created devices with which to deliver them. In March, the company signed a licensing deal giving Cedars-Sinai Medical Center in Los Angeles rights to use Neuralstem's platform and floating cannula in its research into spinal cord injuries and chronic diseases.
In January, Neuralstem received FDA approval to try the same spinal cord cells on patients with chronic spinal cord injury leading to complete paralysis. The cells will be given one-to-two years post-injury. This week, University of San Diego neurologist Martin Marsala reported Neuralstem’s cells significantly alleviated some motor and sensory damage in rats with acutespinal cord injury.3
Using its cells to screen for drugs, the company developed four neurogenic small molecule compounds for the treatment of major depressive disorder. An FDA-approved Phase 1b safety trial of one drug is ongoing.
A stroke clinical trial using Neuralstem cells will soon be launched in China.
“We’re there and it’s real,” says Garr of the stem cell regeneration era.
References
1. Glass, J.D., et. al. “Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients,” Stem Cells, Vol 30, Iss 6, June 2012: p1144-1151.
2. Ryugo, D.K. et. al. “Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry,” The Journal of Comparative Neurology, Vol 514, Iss 4, Jun 1, 2009:p297-309.
3. Van Gorp, S., “Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation,” Stem Cell Research & Therapy,” Vol 4, Iss 5, May 28, 2013: p57.
Further Reading
1. Raore, B., et. al. “Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs,” Spine, Vol 36, Iss 3, February 1, 2011: E164-171.(This manuscript describes the use of human spinal stem cells in a large animal model to support translation to the current human trial.)
2. Lunn, J.S., et. al. “Stem cell technology for the study and treatment of motor neuron diseases,” Regenerative Medicine, Vol 6, Iss 2, March 2011: p201-213. (This manuscript details advantages of stem cell therapies for motor neuron diseases including ALS and presents an overview of the Phase 1 trial.)
3. Lunn, J.S., et. al. “Stem cell technology for neurodegenerative diseases,” Annals of Neurology, Vol 70, Iss 3, September 2011:p353-361.
4. Boulis, N.M., et. al. “Translational stem cell therapy for amyotrophic lateral sclerosis,” Nature Reviews Neurology, Vol 8, Iss 3, December 13, 2011: p172-176. (The above two manuscripts describe stem cell therapy and the FDA-approved Phase I trial of human spinal stem cells in patients with ALS.)
Follow Cynthia Fox on twitter at @cynthfox.





