Marburg, Ebola’s Relative, Cured in Monkeys
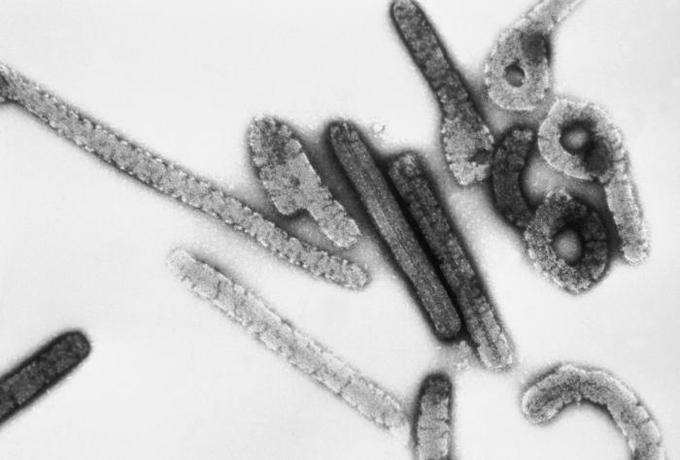 An experimental drug saved the lives of 16 of 16 monkeys with the Marburg virus, a killer near-indistinguishable from Ebola, just as symptoms broke out, said a new study.
An experimental drug saved the lives of 16 of 16 monkeys with the Marburg virus, a killer near-indistinguishable from Ebola, just as symptoms broke out, said a new study.
Thomas Geisbert, senior author of the new Science Translational Medicine study, said treatment with an siRNA molecule, wrapped in a lipid nanoparticle, saved macaque monkeys even when administered on Day 3 post-infection, when animals began showing symptoms. All control animals who did not receive the Tekmira drug— TKM Marburg— died between days seven and nine. SiRNA keeps the virus from replicating by binding to messenger RNA.
Geisbert, a University of Texas microbiologist, said at a press conference that this is the first time one drug saved animals from Marburg days after the virus infected them. Right now, the only way to detect a Marburg or Ebola infection in humans is to wait several days, until the virus reaches detectable blood levels. A similar drug by the same company— TKM Ebola— has been found to work on monkeys when given 30 minutes post-infection.
RELATED: Two Anti-Ebola Vaccines in Historic Race
The promise of such experimental drugs is crucial in light of the ongoing Ebola outbreak in West Africa and the appearance of Marburg in Uganda. On Oct. 6, Uganda's Ministry of Health confirmed that a Ugandan health worker recently died of Marburg, which typically manifests as a viral hemorrhagic fever. The Associated Press reported that another man— who was initially suspected of having hemorrhagic fever— was awaiting tests to confirm Marburg virus, and an additional 80 health workers who had been in contact with the victim were being held in quarantine.
Meanwhile, the first Ebola patient diagnosed in the United States died in a Dallas, Texas, hospital Oct. 8. More than 3,400 people have been killed this year by the Ebola outbreak in West Africa and a nursing assistant contracted the disease in Spain after caring for an Ebola victim there.
The World Health Organization (WHO) previously said the current Ebola/Marburg emergency warrants the use of experimental drugs, without further trials, when/as needed. “I would have no problems taking this product myself,” Geisbert said.
Some dispute
There is some dispute, however, over the main attraction of TKM Marburg; that is, the claim that it works as the first symptoms appear. While the Geisbert team reported that on Day 3 their monkeys began to show symptoms, Tulane University tropical disease specialist Daniel Bausch disagrees. Bausch left Sierra Leone in July, only a few days before a Tulane colleague—Sheik Umar Khan— contracted Ebola while treating patients, and later died.
“Average incubation time in humans is eight to 10 days. Less in monkeys, but still greater than three days,” says Bausch, who was not involved in the Science study. “If you look at the paper, the control monkeys started having symptoms around five or six days. The paper and results are promising. The later a drug or vaccine can work after exposure the better. But of course you ultimately want something that cures somebody after the onset of symptoms, which is when people are going to present for care. This treatment might do that, but we just can't know from the study that was done. It's not really a criticism, but rather just the limits of conclusions that can be drawn from this particular study design. I'm sure that the authors would agree.”
However, Geisbert, also by email, says the viral doses to which his team exposed his non-human primates ranged from 10,000 to 30,000 pfu (plaque forming units) depending on the strain or species of the filovirus. “This is extremely high,” he said.
The reason? The ethical issues and expenses behind non-human primate studies result in small group sizes. To get statistically significant numbers “we cannot afford to have even a single control animal survive,” Geisbert said. (Mouse/guinea pig studies are done with ten or more per group.)
By giving such high doses, “we shorten the disease course so the non-human primates succumb faster than what you typically see in filovirus-infected humans. So, Day 3 in our non-human models would likely represent a much later point in time in humans,” he said.
RELATED: ZMapp: Best Anti-Ebola Treatment Yet
The best comparison in humans is the 1976 outbreak of Ebola-Zaire, where needles were reused, Geisbert said. “There were 85 confirmed needle sticks, and all 85 people died and the disease course was much faster than most cases. That is more what our non-human primate models represent…nowhere near what normally happens in most natural exposures where the doses are likely much lower, and not by direct injection.”
Furthermore, Geisbert said, he prefers the word “signs” to “symptoms.” Symptoms imply things “felt and communicated.” Signs include detectable virus levels. “Non-human primates cannot tell you that they are feeling a little off at an early stage of illness. When humans are at a point where virus can be detected, there are usually some type of symptoms that include things like body aches, myalgia, malaise or headache. So, I don't necessarily agree that you can detect virus in humans that much ahead of feeling really bad. By analogy, the non-human primates that were viremic [three of four in the Science study at Day 3] would very likely have had some of these symptoms. We can't put this into a scientific paper, but we watch the animals for many days before a study starts. While they cannot tell us they are not feeling well, it was obvious to us that all four in the Day 3 group were not up to par on Day 3,” he said.
In the Science study, the 16 monkeys receiving Marburg-TKM were divided into four groups treated at different times: 30 to45 minutes after infection and at one, two or three days after infection. Four were given the drug three days after exposure.
Said Geisbert: “Where I very much disagree with Dan [Bausch] is that if you have a situation in an outbreak where you know that you have possible contact exposures, and you closely monitor those people, including doing real time PCR, you can then treat them at the onset of signs— most importantly, the detection of virus. What we show in this paper is that when you treat at the onset of signs, you protect the animals. This becomes really important when you have limited [supply of] drugs like ZMapp, or these siRNAs, where you want to make the best use of them.”
The experimental ZMapp, by the company Mapp Pharmaceuticals, has been given to a handful of patients and saved most, although a priest on the drug died in Madrid after being airlifted from Liberia. The last known doses of ZMapp were sent to Liberia on Aug. 13, and were used to treat three Liberian doctors. One of those doctors has died from the virus and ZMapp supplies are now depleted.
In summary, Geisbert said, the disease course in their study’s monkeys was much faster than in humans, so “you likely have a lot more time.” And, he said, he believes human contacts can often be monitored closely and that treatment of contacts can play a key role in managing outbreaks “both in terms of spread, and also morale, in that people are more likely to report something if they know there is something that can save them.”
In July, the FDA put a hold on the clinical trial of related drug TKM-Ebola, to ask some questions. The doses in the trial were higher than in monkeys, and there was some nausea. But, as noted, the WHO recently gave the green light for the drug to be used, on an emergency basis, in patients.
Marburg and Ebola are currently circulating in the same countries. In 2004-2005, over 90 percent of those stricken with Marburg died. It is reported that more than 1,400 people in West Africa have died of Ebola in recent weeks.

