Investigating Cell Death Pathways Using Simplified Cytometry
 The study of cell death mechanisms and pathways has become increasingly complex over the years with the improved understanding that processes such as apoptosis, autophagy, necro-apoptosis and others can act singly or in concert to modulate cell death. Further, the process of distinguishing mechanisms at play has also become more complicated with the revelation that indicators/biomarkers typically characteristic of a particular mode of cell death also trigger or influence other death mechanisms. It has thus become even more critical to understand the changes the cell has undergone by evaluating information on multiple cell health and cell death indicators to truly understand the sequence of events at play.
The study of cell death mechanisms and pathways has become increasingly complex over the years with the improved understanding that processes such as apoptosis, autophagy, necro-apoptosis and others can act singly or in concert to modulate cell death. Further, the process of distinguishing mechanisms at play has also become more complicated with the revelation that indicators/biomarkers typically characteristic of a particular mode of cell death also trigger or influence other death mechanisms. It has thus become even more critical to understand the changes the cell has undergone by evaluating information on multiple cell health and cell death indicators to truly understand the sequence of events at play.
Several processes and markers are studied to understand cellular health status. These include: changes that appear earlier such as oxidative stress or mitochondrial membrane depolarization that are common to multiple pathways; activation of individual members of the family of caspases; flipping of phosphatidylserine as detected by annexin V binding; and DNA fragmentation and cell membrane permeabilization. In addition, markers of autophagic processes, DNA damage, etc. can also provide different layers of information that further our understanding of the cell death process. Time and dose response study of the modulation of these changes can provide critical insights into the sequence of the events in the pathway and allow a more comprehensive picture of the changes taking place.
While many technologies, ranging from western blots, to imaging methods, to cytometry, are available to characterize cell death, flow cytometry has become and remains a very powerful method for these studies. In combination with advances in chemical biology and pathway analysis, flow cytometry can provide a deeper insight into molecular markers and cellular changes. Flow cytometry can provide multiparametric “in-cell data” rather than average response. Statistically relevant data can be acquired on thousands of cells rapidly, and there is minimal operator bias in the acquisition and quantification of data. While flow cytometry was traditionally the domain of the experts and was relatively expensive and sophisticated, making access to the technology difficult, recent evolution of low-cost, ultra-compact multiparameter flow cytometers has made it possible and accessible for many to perform such studies routinely and on their benchtop with minimal help.
The Muse® Cell Analyzer (EMD Millipore, Billierica, MA) is a low-cost, simple-to-operate cytometry platform based on microcapillary flow that can provide quantitative cellular information to overcome the challenges described above. The instrument is a closed platform, where the reagents and software are closely paired together for dedicated applications, thus simplifying cellular analysis. The microcapillary flow system results in reduced cellular sample consumption and very low generation of biohazardous waste. The system yields percentages, counts and fluorescence intensities of populations.
Establish your company as a technology leader. For 50 years, the R&D; 100 Awards, widely recognized as the “Oscars of Invention,” have showcased products of technological significance. Learn more.
The platform provides multiple assay solutions for exploring cell death mechanisms, including early and late stages of apoptosis and other cell death processes, such as oxidative stress, nitric oxide stress, mitochondrial potential changes, annexin V response, and caspase activation. In addition, it also offers the capability to study other questions of importance to cell health, such as cell cycle or cell proliferation and autophagy. Many of these are two-color assays that allow study of two markers simultaneously.
 An example of a cell health assay on analyzer is the Muse® MitoPotential Assay (Figure 1). This is a simple, no-wash assay that provides percentage and concentration of cells demonstrating mitochondrial membrane depolarization and/or cell death. The assay is based on detection of mitochondrial membrane potential depolarization using a cationic lipophilic dye along with a dead cell dye that enters cells with compromised membranes. Depolarized cells are detected by a decrease in fluorescence. Changes in mitochondrial membrane potential can be common to multiple pathways of cell death and hence its assessment is critical to many cell death studies.
An example of a cell health assay on analyzer is the Muse® MitoPotential Assay (Figure 1). This is a simple, no-wash assay that provides percentage and concentration of cells demonstrating mitochondrial membrane depolarization and/or cell death. The assay is based on detection of mitochondrial membrane potential depolarization using a cationic lipophilic dye along with a dead cell dye that enters cells with compromised membranes. Depolarized cells are detected by a decrease in fluorescence. Changes in mitochondrial membrane potential can be common to multiple pathways of cell death and hence its assessment is critical to many cell death studies.
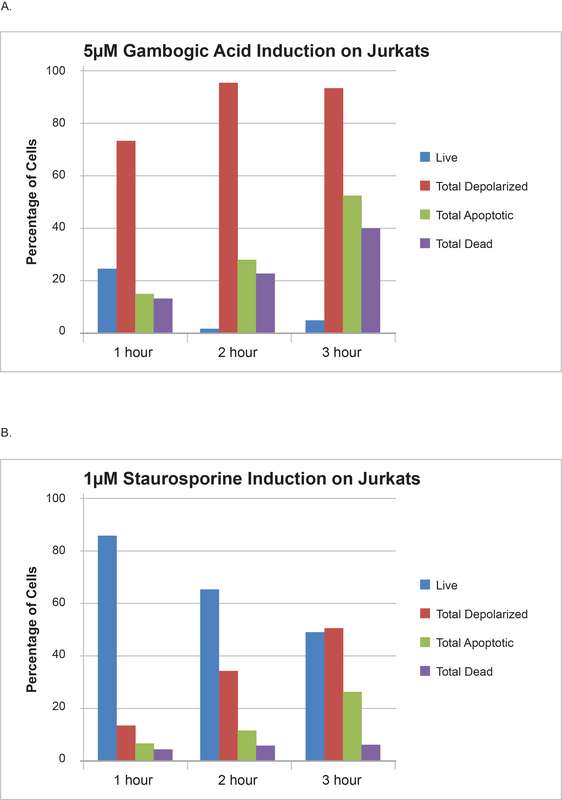
The combination of cell health assays with kinetics and dose response studies can provide more enriched information and significant insights into the compound mode of action. Figure 2 summarizes data from a study in which Jurkat cells were treated with anti-cancer drugs gambogic acid (A) and staurosporine (B) for different periods of time and then analyzed.
As the figure reveals, treatment with gambogic acid results in a rapid and dramatic change in the percentage of cells showing mitochondrial depolarization with slower kinetics being observed for apoptotic response with annexin V and cell death through the time course. Treatment with staurosporine, on the other hand, results in slower and parallel changes in mitochondrial potential depolarization and apoptosis responses. The data thus suggests that gambogic acid causes a severe and rapid impact on mitochondrial membrane potential. This is consistent with literature publications that suggest that gambogic acid actually targets and localizes to the mitochondria and triggers the intrinsic pathway of apoptosis and is thus a “mitocan.” This kind of information might have been missed without the study of the time-based response of treatment with multiple cell health assays.
Technologies like this can help reduce the analytical complexity and facilitate obtaining cytometric-based data on cell health and cell death aspects with ease and empower greater numbers of researchers to elucidate pathways of cell death in their studies. A deeper understanding of triggering mechanisms and processes that lead to cell death can further our understanding of a wide range of diseases and enable the discovery of better drugs and strategies to modulate them.




