Transfection Tips and Tricks
1.Introduction
Transfection is used to modify cells for a wide range of applications, from basic research looking to understand gene function, to establishing disease models, developing new therapeutics, bioprocessing and biomanufacturing. The process hinges upon transferring a substrate into a cell to achieve a desired outcome. This substrate is often DNA or RNA, although proteins and other molecular complexes can also be transfected into cells. Several methods of transfection exist, including lipofection, electroporation and viral transduction.
Lipofection involves encapsulating the substrate in a lipid-based vesicle, which then enters the cytoplasm via endosomal uptake and is subsequently expressed following cell division and nuclear entry. Electroporation is a physical method that uses an electric field to render the cell membrane permeable. This allows the substrate to enter the cytoplasm and, to some extent, the nucleus too. Variations on this method, such as nucleofection, provide enhanced delivery of DNA and RNA substrates through the nuclear membrane, increasing efficiency in hard-to-transfect cells and allowing faster, more effective expression. Viral transduction entails packaging a DNA or RNA substrate in a viral particle, which then ‘infects’ the host cell to deliver the substrate.
Taking into consideration the many method-, cell- and substrate-specific requirements of different applications, it is important to create optimal transfection efficiency, cell viability and experimental reproducibility. In order to offer better chances of success, researchers working with transfection might benefit from considering the following advice.
2.General transfection tips
2.1.Cells
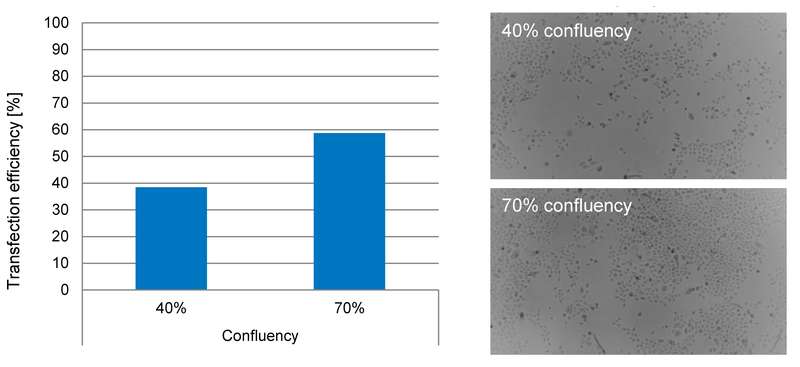
Cell type and culture conditions can significantly affect transfection efficiency (see Table 1). For example, cell confluency can play an important role. However, this is often a subjective measurement, so it is advisable to use a cell imaging device to monitor cells and standardize the process so that transfection efficiency can be optimized (e.g. see Figure 1). Cell source can also influence transfection efficiency, which may vary between primary cells taken from different patients or different cell line clones. It is recommended that early passage cells are used, as cell properties may change after extensive passaging. Lastly, contamination can also impact transfection efficiency. For example, mycoplasma cannot be seen under the microscope and is often overlooked. Regular screening is therefore advised and contaminated cells should not be used.
Table 1. Common cell and substrate considerations when carrying out a transfection.
| Cell recommendations: |
|
- Cells should be in the logarithmic growth phase |
| Substrate recommendations: |
|
- The use of highly purified DNA is recommended |
2.2.Substrates
The purity and quality of the substrates in use are important determinants of transfection efficiency (see Table 1). Supercoiled DNA is the most effective substrate form when using DNA, as nicked or open circle DNA may lead to lower efficiency. It should also be noted that substrate quality may be compromised as a result of the age of the plasmid preparation or multiple freeze/thaw cycles. As a preliminary precaution, a gel can be run to confirm substrate quality.
The plasmid backbone can also impact results. For example, some promoters provide stronger expression levels than others. Furthermore, if more than one gene is under the influence of the same promoter in a bicistronic plasmid, the order can affect expression. In this case, downstream genes may be expressed at lower levels and thus may require more sensitive detection in order to have reliable and accurate analysis.
Lastly, an mRNA substrate may be a better option for DNA-sensitive cells or where limited protein presence is beneficial. However, mRNA is prone to cellular degradation, so it should be capped and polyadenylated in order to prevent this. The optimal amount will also need to be titrated, which may be higher than that used for plasmids.
3.Application specific considerations
3.1.Reporter gene assays
It is important to consider how and when to measure reporter gene expression in order to produce insightful data. For example, the time point of maximal expression is dependent on when gene expression starts and the protein half-life. Factors that influence the optimal time of analysis can impact quantification.
 Analysis of the reporter gene often involves measuring the transient expression of a fluorescent protein. The signal intensity per cell can vary depending on the fluorescent protein used and its distribution in the cell. Therefore, in order to avoid underestimating assay efficiency due to low reporter signal intensity, the use of a more sensitive method of detection is recommended, such as flow cytometry.
Analysis of the reporter gene often involves measuring the transient expression of a fluorescent protein. The signal intensity per cell can vary depending on the fluorescent protein used and its distribution in the cell. Therefore, in order to avoid underestimating assay efficiency due to low reporter signal intensity, the use of a more sensitive method of detection is recommended, such as flow cytometry.
3.2.Co-transfection
When transfecting a cell with multiple substrates, it is important to ensure that they are of high quality and purity since the total DNA amount transfected might be higher than usual with just a single plasmid. Plasmid size, promoter strength, and the complexity of the gene product should also be taken into account and the plasmid ratio optimized. These considerations are especially important when reprogramming induced pluripotent stem cells or carrying out genome editing.
3.3.Stable integration
Prior to starting any experiment, it is important to decide upon a clonal strategy. For example, polyclonal selection involves bulk batch culture, whereas monoclonal selection involves a limiting dilution or semisolid medium. When using antibiotic resistance as a means of selection, kill curve analysis should be conducted to define the optimal selective pressure. Ideally, cells should be allowed a post-transfection recovery period and the same antibiotic batch should be used throughout. Lastly, seeding density should be determined prior to conducting the stable experiment.
The amount of plasmid required depends on the cell type. In addition, linearization of the plasmid may prove advantageous and help to achieve a higher percentage of resistant and positive clones. It’s also important to note that, after generation of the stable clone, gene expression may be silenced by methylation. To prevent this, additional subcloning and expansion in high antibiotic concentrations may be required.
4.Conclusion
There are a number of important factors to consider when designing a transfection experiment. Thorough and meticulous planning that takes into account the substrate, cell type, application, transfection method and the overall goal of the experiment, will significantly help researchers achieve transfection success.




