Enhanced Analysis of Extracellular Vesicles with Imaging Flow Cytometry
Once thought of as cellular debris, extracellular vesicles are now recognized as key messengers that mitigate cell-cell communication. These tiny membrane-bound packages of macromolecules, which include microvesicles, exosomes, and apoptotic bodies, can form in one of two ways: intracellularly by invagination of the plasma membrane, or by budding (or shedding) directly from the cell membrane into the extracellular space. Extracellular vesicles are secreted by m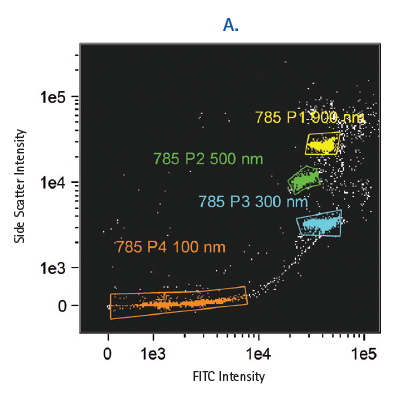 ost, if not all, human cell types in response to activation, injury, or apoptosis, and may serve as the primary means for transfer of genetic material and signaling molecules between cells in interstitial spaces and via biological fluids, including plasma, cerebrospinal fluid, sputum, and milk.
ost, if not all, human cell types in response to activation, injury, or apoptosis, and may serve as the primary means for transfer of genetic material and signaling molecules between cells in interstitial spaces and via biological fluids, including plasma, cerebrospinal fluid, sputum, and milk.
Extracellular vesicles are believed to serve as key mediators in the pathogenesis of neurological, vascular, hematological, and autoimmune disease. Moreover, extracellular vesicles are also considered vital intermediaries in cancer processes that contribute to poor disease outcomes, such as metastasis. The translation of extracellular vesicle research to the clinic to aid in the diagnosis and treatment of many disease types will require the development of standard assays for their characterization and quantification. The challenge of such analysis lies in the submicron size (generally 50-1000 nm in diameter) of each vesicle, as well as the complexity of the biofluids in which they are typically suspended. While sensitive enough to resolve tiny cellular particles, high-magnification microscopy is limited by the typical dispersal of microvesicles in biofluids and a lack of quantitative data. Non-imaging flow cytometers can measure objects in suspension, but are generally unable to detect particles less than 400 nm in diameter. In addition, these instruments cannot provide visual confirmation of particle integrity for characterization.
As a result of the above-mentioned challenges, efforts to achieve consensus on the analysis of extracellular vesicles are hindered, and concerns about data comparability are being raised within the scientific community. The recent characterization and quantification of extracellular vesicles has been achieved, however, using imaging flow cytometry, which suggests a promising role for this technology in the advan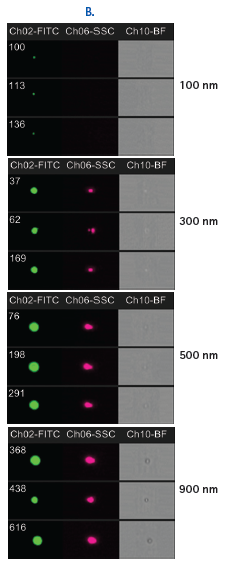 cement of this field.
cement of this field.
Reliable quantification of extracellular vesicles
Through precise fluid mechanics, ultra-sensitive fluorescence detection, and software engineering that facilitates quantitation and statistical analysis, Amnis imaging flow cytometers (EMD Millipore) overcome the technological barriers that traditional flow cytometers face in the analysis of extracellular vesicles. The enhanced sensitivity of this technology for fluorescence detection has been demonstrated by exceptional resolution of mixed peripheral blood mononuclear cell subpopulations in heterogeneous samples (data not shown). Charge-coupled device (CCD) camera acquisition of fluorescent signal confers increased sensitivity and facilitates the resolution of less common populations.
For non-imaging flow cytometry, gating relies on the degree of light scatter and fluorescence intensity, which can result in frequent, inadvertent inclusion of contaminating particles in the presumed extracellular vesicle population. A key feature of imaging flow cytometry, however, is the pairing of fluorescence intensity data with an image for every event collected, providing functionality that allows the user to distinguish true single particles from aggregates and cellular debris. This pairing feature also creates searchable scatter plots and image galleries that can facilitate visual confirmation of fluorescent events.
The Amnis ImageStream is equipped with a 60X objective, which allows for the detection and characterization of extracellular microparticles. The barriers to resolving individual microvesicles in biofluids are addressed by exceptionally precise hydrodynamic focusing, which reliably segregates extracellular vesicles as individual acquisition events. The CCD-based detection has been validated for the detection and analysis of submicron particles that are significantly smaller than the 400-500 nm threshold demonstrated by even the most sensitive photomultiplier (PMT)-based cytometers using fluorescent intensity scatter plots and images of FITC-conjugated beads (Figure 1). In addition, isolated and cell-bound fluorescently labeled exosomes have been detected using this technology, and the degree of internalization of exosomes measured using the software internalization feature (Figure 2).
Moreover, the acquisition software automatically records the event count and volume acquired per sample. The number of extracellular vesicles per μL can therefore be  readily displayed without the need for costly and/or laborious optical, electron microscopy, or enzyme linked immunosorbent assay (ELISA)-based methods. This capability also addresses the recommendations of the International Society for Extracellular Vesicles regarding the standardization and reduction of workflow in extracellular vesicle preparations, collection, and analysis.
readily displayed without the need for costly and/or laborious optical, electron microscopy, or enzyme linked immunosorbent assay (ELISA)-based methods. This capability also addresses the recommendations of the International Society for Extracellular Vesicles regarding the standardization and reduction of workflow in extracellular vesicle preparations, collection, and analysis.
Finally, sample volume and preparation time are greatly reduced. Vesicles, cells, and beads in heterogeneous suspension can be differentiated by side-scatter and morphology characteristics, thereby eliminating the need for extracellular vesicle isolation prior to flow acquisition.
Taken together, these innovations in flow cytometry enable a new standard for the consistent detection, quantitation, and characterization of extracellular vesicles. These observations also suggest a promising new role for imaging flow cytometry in the advancement and clinical translation of extracellular vesicle biology.



