Cancer Chain in the Membrane
Supercomputer simulations have shown that clusters of a protein linked to cancer warp cell membranes, according to scientists at The University of Texas Health Science Center at Houston (UTHealth) Medical School. This research on these protein clusters, or aggregates as scientists call them, could help guide design of new anticancer drugs.
"The aggregate is a large substructure that imposes some kind of curvature on the membrane—that's really the major observation," said Alemayehu Gorfe, assistant professor of Integrative Biology and Pharmacology at the UTHealth Medical School.
Gorfe led the study published in the Journal of Physical Chemistry Letters in April 2014. They created coarse-grained molecular dynamics simulations of the Ras protein using the Lonestar and Stampede supercomputers at the Texas Advanced Computing Center, one of the nation's leading academic supercomputer centers and part of The University of Texas at Austin.
"Without these two machines, the work in this paper and our other recent publications would not have been possible. TACC was key to this project," Gorfe said. The University of Texas System Research Cyberinfrastructure (UTRC) facilitated the computing resources used by his research group. The National Institutes of Health (NIH) funded the study.
More than one-third of all human cancers are associated with somatic, or post-conception, mutations in Ras proteins, according to the NIH. "Mutations on one of the Ras proteins, Kristen or K-Ras, are responsible for 90 percent or more of pancreatic cancer cases," Gorfe said. "It tells you that it is a very, very important anticancer drug target."
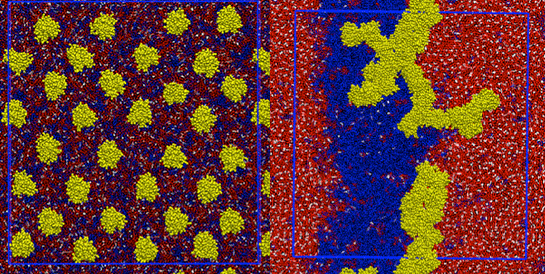 The Ras gene, which codes for the Ras proteins, was discovered in the 1960s, and represents the first gene identified with the potential to cause cancer in humans. Ras gets its name from the rat sarcoma virus, where it was first identified. Scientists believe Ras proteins mediate a chain of cell signals that can cause out of control cell reproduction and ultimately cancer. Scientists today have little understanding of how or what happens when Ras proteins form small, nano-sized clusters on the membrane. The researchers hope that a better understanding of how Ras proteins cluster together and interact with other proteins in cells will bring them one step closer to developing therapeutic targets for Ras-driven tumors.
The Ras gene, which codes for the Ras proteins, was discovered in the 1960s, and represents the first gene identified with the potential to cause cancer in humans. Ras gets its name from the rat sarcoma virus, where it was first identified. Scientists believe Ras proteins mediate a chain of cell signals that can cause out of control cell reproduction and ultimately cancer. Scientists today have little understanding of how or what happens when Ras proteins form small, nano-sized clusters on the membrane. The researchers hope that a better understanding of how Ras proteins cluster together and interact with other proteins in cells will bring them one step closer to developing therapeutic targets for Ras-driven tumors.
"It is these nanoclusters, these transient substructures on the cell membrane that assemble and disassemble quickly, that are involved in signal transmission," Gorfe said.
Gorfe's computer simulations showed Ras proteins cluster together, or form aggregates, on the cell membrane. And this led him to question what they might do there.
"Would the proteins just sit passively and not affect the membrane at all? Or would they somehow change its shape? That is the question we asked," Gorfe said. "The observation was clearly that they have a major effect on the membrane."
When the normally flat bilayer of the cell membrane gets drastically curved, scientists call it membrane remodeling. Membrane remodeling typically occurs during cell division, during cell movement as it slithers around, and during vesicle formation, when a small piece buds off like what you might see in a lava lamp.
On a smaller scale, the membrane folds and buckles near clusters of Ras proteins. Gorfe said that understanding the dynamics of Ras proteins is critical to its study as a potential drug target for cancer.
"One of the major contributions, in my view, of molecular dynamics simulations is the information we get using this method about the dynamics of biomolecules in general," Gorfe said.
What's more, simulations are useful for structure-based, computer-aided drug design. "We try to use these snapshots of the moving protein, or an ensemble of structures, to dock small molecules in a virtual screening setting," Gorfe explained. Docking refers to the process of a drug molecule attaching itself to the surface of a protein. "Of course, if we develop inhibitors starting with these kinds of studies that would help treat cancer, that would be wonderful."
Priyanka Srivastava researches the Ras protein with Gorfe in his lab as a Keck Postdoctoral Fellow at UTHealth. "Our ultimate goal is to identify novel pockets that transiently open during protein motion but are hidden in the average experimental Ras structure so that we can target those," Srivastava said.
For more than 40 years, scientists have known that Ras is a cancer-causing protein but have been unable to stop it. Ras acts like a switch that must be turned on for cells to reproduce. The problem is that when RAS genes get mutated, that switch just won't turn off, and that leads to out of control cell growth.
"In the 1990s, there was a lot of excitement about a class of compounds that inhibit one of the enzymes that puts lipids on Ras proteins," Gorfe said, "and lipidation is important for membrane binding of the protein. The thought was, if we inhibit one of the enzymes that actually puts lipids on Ras, we might dislodge it from the membrane surface, or we might be able to prevent it from membrane binding, and in so doing prevent its function."
These compounds failed because Ras is tricky. "If you knock out one of the enzymes that it uses, it just finds another to bind itself to the membrane," he explained.
For this and other reasons, Ras was considered undruggable, according to Gorfe. Interest in Ras as an anticancer drug target faded. Hope in targeting Ras has sprung up in the last five years due in large part to simulations and analyses enabled by supercomputers.
"We learned quite a bit about the importance of dynamics in drug discovery in general and for Ras proteins, in particular," Gorfe said. "The dynamics came into the picture."
Understanding the dynamics will help scientists reveal the vulnerable spots of the Ras molecule, according to Srivastava.
"Using TACC resources, we carried out an atomic-level simulation of membrane-bound K-Ras. And it was very interesting that it actually revealed new binding sites that had not been previously characterized. At this point in time we are doing site-directed virtual screening against these sites, which may result in some sort of promising anticancer drug."
"Uncovering new membrane binding sites on Ras may lead to a greater understanding of how its on-off switch is controlled and how to specifically inhibit the unregulated cell growth in cancers caused by defective Ras," said Jean Chin, program director in the Division of Cell Biology and Biophysics of the National Institute of Health's National Institute of General Medical Sciences, which funded the research. "These simulations by Dr. Gorfe will also provide new models and ideas for his colleagues and other biologists to test experimentally."
Another advance was the realization earlier by Gorfe in collaboration with J. Andrew McCammon of the University of California at San Diego and Barry Grant of the University of Michigan, as well as other scientists, that Ras is what's called an allosteric enzyme.
"What that means is that binding of small molecules further away from the part of the protein that's actually involved with the interaction with other proteins or substrates—that's what you want to prevent—impacts interaction via other parts of the protein."
Simulations—and computational modeling in general—have become an integral part of modern research in biology, according to Gorfe.
"In some cases, information that you would not be able to get from experimental methods alone can be obtained from simulations. Therefore, simulations can combine with experiments to provide detailed insights into how Ras proteins work in the cell. In this regard, we are fortunate to have a long-standing collaboration with experimental biologists, and I would like to especially thank Dr. John F. Hancock and members of his laboratory here in our school. With the level of advances in algorithms, infrastructure, and computer speed, we have begun to ask more fundamental questions and to use more complicated model systems," he added.
And for the millions of people worldwide who suffer from Ras-associated cancers, those answers couldn't come sooner.
Source: Texas Advanced Computing Center (TACC) at The University of Texas at Austin





