‘Remarkable’ Therapy Makes Old Mice Young, May Hit Clinic This Year
 A futuristic anti-aging approach, variously described and utilized by three different Harvard and Stanford groups this week, may hit the clinic by year’s end.
A futuristic anti-aging approach, variously described and utilized by three different Harvard and Stanford groups this week, may hit the clinic by year’s end.
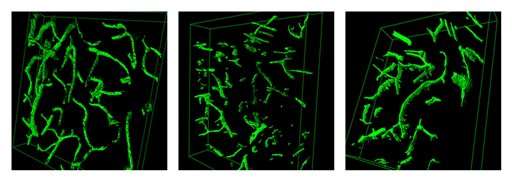
The research all began with “heterochronic parabiosis.” That is, old mice were hooked up to young mice via their circulatory systems— and experienced tissue rejuvenation. Harvard teams then winnowed out of young blood a protein called growth differentiation factor 11 (GDF11). In mice, this factor appeared to reverse aging in body and brain, generating new blood vessels, muscles, and neurons. One study reported cognitive gains.
“In terms of the neurogenesis, I was surprised, first of all, that parabiosis and GDF11 had any effect on the central nervous system (CNS),” Harvard University neuroscientist Lee Rubin told Bioscience Technology by email. Rubin was senior author on a Science paper this week detailing the effect of parabiosis and GDF11 on the brain. His crew found that GDF11, injected into the brain, improved rodents’ sense of smell following an increase in olfactory neurons and blood vessels.
“Because of the presence of the blood-brain barrier, there were no guarantees that circulating systemic factors would alter CNS function. In our studies, however, we showed that at least some of the effects are on the vasculature, and this doesn't require that the active factors enter the brain,” Rubin said.
The second great surprise, Rubin said, was the fact that “one single factor, GDF11, can improve the function of multiple tissues in the same mice. Most people, including scientists, would not have expected this, although researchers who study the aging process may not be as surprised by our findings.”
Finally, Rubin said, as someone who works on neurodegenerative disease, and whose lab focuses on preventing brain cells from malfunctioning and dying, “I found it remarkable that brain function can be improved even after degenerative processes associated with aging have occurred.”
Harvard stem cell researcher Amy Wagers started it all in recent years, working since 2000 with parabiotic mice to find GDF11. She told Bioscience Technology via email her group is now “trying to determine whether GDF11 serves as a ‘youth maintenance factor’ as well as a restoration factor for aged tissues. Do young animals need to maintain high levels of GDF11 to stay ‘young’?”

Mechanism of action
Wagers wrote the second Science paper out this week on GDF11. Her team last year proved, with Harvard’s Richard Lee, that GDF11 reduces cardiac hypertrophy in mice. (See earlier Bioscience Technology story.) In the recent paper, she found GDF11 improved strength and endurance of skeletal muscles—again, after she found a similar effect via parabiosis.
Wagers told Bioscience that more than just new gene expression may be at work. DNA repair may be at work. “We are really just beginning to understand what's going on in terms of GDF11's effects on DNA repair,” she said. “We see evidence of increased damage to DNA in aged muscle stem cells (‘satellite cells’), and we see that damage resolved after exposure to GDF11. The resolution of the damage is associated with a restoration of the differentiation activity of the stem cells: they are more able to produce mature muscle cells needed for muscle regeneration. One possibility is the accumulated damage prevents muscle stem cells from differentiating, and GDF11-stimulated resolution of the damage allows it. Another is that accumulation of ‘damage’ is an indicator of stalled differentiation, and GDF11 overcomes this differentiation block and thus allows damage resolution. Our future experiments are aimed at distinguishing between these two possibilities, and will clarify GDF11's mechanism of action more completely.”
It is unknown if the approach extends lifespan. The group has not assessed that. “We are focused really on ‘healthspan,’ maintaining the healthy years of life,” Wagers said.
She wants to understand “why GDF11 is lost with age; where it is produced; whether its loss reflects a loss of the cells that make it, or a reduction in their production capacity.” This will help her determine the best way to target GDF11 for therapy.
The work is “aimed at both revealing fundamental mechanisms and pathways that control aging, and at developing treatments for age-related diseases (such as muscle wasting, neurodegeneration and heart disease), which we now appreciate could be aided by a common strategy to boost levels of GDF11 in the blood,” Wagers said.
Wagers is talking to venture capital firms. But before GDF11 hits the clinic, it needs modification, as too much of is needed per patient, she has reported.

Clinic bound?
The third rejuvenation paper out this week details an approach that may be clinic-ready by year’s end. Published in Nature Medicine, the paper describes the work of Stanford University neuroscientist Tony Wyss-Coray. Like the Harvard crew, his group exposed older mice to parabiosis. But they followed that up by simply infusing, into a different group of older mice, the plasma of younger mice— blood minus cells and platelets— bypassing parabiosis. Both approaches resulted in neurons sprouting new dendrites in the hippocampus. Both approaches resulted in improved spatial memory, and improved fear response.
Because plasma transfusion is already practiced in hospitals, it does not need FDA approval. So the Wyss-Coray team hopes to start treating a few Alzheimer’s patients with the plasma of younger people by year’s end, according to published reports. The company that may guide this project is called Alkahest, with which Wyss-Coray is affiliated.

