Avoiding Contamination in Tissue Culture Plastics
LISTED UNDER:
Tissue culture is a vital element of many research laboratories. Extensive experiments often stem off cultured cells and provide valuable insight. Growth of unwanted microbes and mold in tissue culture plastics impacts and pauses all future experiments. The researcher must then use valuable time and resources to obtain new cultures and avoid contamination in the future. Tissue culture plastics include flasks, plates, and dishes. Contamination is not only costly, but results in great productivity loss. TPP, a manufacturer of flasks, plates, and dishes and distributed in the United States by MIDSCI, addresses each contributing issue to contamination introduced by tissue culture plastics with its innovative and unique design.
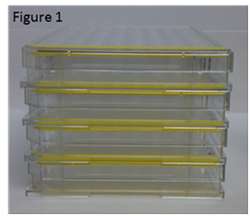
One of the biggest obstacles to overcoming and avoiding contamination is removing the optimal microbe breeding grounds. In order for microbes to grow, they need heat, a moist environment, and sustenance all provided by Incubators. Condensation contributes to the moist environment and often builds up between stacked dishes, plates, and flasks. TPP dishes, plates, and flasks are manufactured with vented stacking feet (Figure 1) decreasing condensation by even air flow and even temperature distribution.
 The “angled deck” or “dead space” seen in some flasks also contributes to microbe and mold growth (Figure 2a). When flasks are moved, media sloshes up onto the “angled deck” providing an ideal breeding ground for microbes and mold. The “angled deck” or “dead space” provides yet another disadvantage: the pipette shaft must go into the neck of the flask to add reagents reach the media (Figure 2b). Contamination can result from the introduction the shaft to the neck of the flask. The flat growth surface to the neck of the flask and 100% useable space of TPP flasks requires only the sterile pipette tip to enter the flask for small liquid additions. The flask’s design overcomes obstacles the “angled deck” presents and aids in contamination prevention.
The “angled deck” or “dead space” seen in some flasks also contributes to microbe and mold growth (Figure 2a). When flasks are moved, media sloshes up onto the “angled deck” providing an ideal breeding ground for microbes and mold. The “angled deck” or “dead space” provides yet another disadvantage: the pipette shaft must go into the neck of the flask to add reagents reach the media (Figure 2b). Contamination can result from the introduction the shaft to the neck of the flask. The flat growth surface to the neck of the flask and 100% useable space of TPP flasks requires only the sterile pipette tip to enter the flask for small liquid additions. The flask’s design overcomes obstacles the “angled deck” presents and aids in contamination prevention.
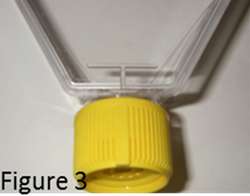 The vented or filtered cap on a flask prevents contamination and allows for varying amounts of air to enter. However, caps only provide a contamination barrier when used and secured properly. Caps can be easily secured improperly, slightly skewed when turned on the neck of the flask. Caps are meant to provide a barrier to outside microbes, effectively sealing microbes out of the flask environment. When a cap of a flask is askew the seal is not intact, allowing microbes within the incubator environment to enter the flask. TPP’s raised ridge area cap ensures proper securing correctly by lining up the ridges. Additionally, the vented caps have audible clicks for proper cap securing (Figure 3).
The vented or filtered cap on a flask prevents contamination and allows for varying amounts of air to enter. However, caps only provide a contamination barrier when used and secured properly. Caps can be easily secured improperly, slightly skewed when turned on the neck of the flask. Caps are meant to provide a barrier to outside microbes, effectively sealing microbes out of the flask environment. When a cap of a flask is askew the seal is not intact, allowing microbes within the incubator environment to enter the flask. TPP’s raised ridge area cap ensures proper securing correctly by lining up the ridges. Additionally, the vented caps have audible clicks for proper cap securing (Figure 3).

Both suspension and adherent cells can be affected by contamination. TPP bioreactors (Figure 4) have sterile gas exchange through the 0. 22µm filter membrane and provide the ideal environment for suspension cells. The five openings of different size within the cap allow for varying levels of gas permeability. The bioreactor, available in 15, 50, and 600 mLs, can be centrifuged directly without transferring, preventing possible introduction of microbes and saving valuable research time.
TPP improves productivity within a lab not only due to the decreased threat of contamination, but also due to the reproducibility of experiments. The even temperature distribution provided by TPP, due in part to the “feet” on the bottom of the flask, is crucial in maintaining reproducibility across wells of a plate. By allowing for proper air flow between the plates and flasks, TPP also decreases evaporation in outside wells of plates, ensuring each well is undergoing the same conditions. TPP saves time and money through its innovative features that decrease contamination and ensure each well is subjected to the same temperature, even when stacked.