Bioprinted 3-D Device Aids Blood Detoxification
 A team of engineers at the University of California San Diego has successfully developed a three-dimensional-printed device, which mimics the operation of the liver to remove dangerous toxins from the blood.
A team of engineers at the University of California San Diego has successfully developed a three-dimensional-printed device, which mimics the operation of the liver to remove dangerous toxins from the blood.
The device, currently designed to be used externally much like dialysis, uses nanoparticles to trap pore-forming toxins that can damage cellular membranes and which are a key factor in illnesses resulting from animal bites and stings, as well as bacterial infections. The UCSD team reported its findings in Nature Communications.
“The concept of using 3-D printing to encapsulate functional nanoparticles in a biocompatible hydrogel is novel,” said Shaochen Chen, a UCSD nanoengineering professor who led the research team. “This will inspire many new designs for detoxification techniques since 3-D printing allows user-specific or site-specific manufacturing of highly functional products,” Chen said.
Nanoparticles have already been shown to be effective at neutralizing pore-forming toxins in blood, but if those nanoparticles cannot be effectively digested they can accumulate in the liver creating a risk of secondary poisoning, especially critical for patients who already are at risk of liver failure. To solve the problem, the UCSD researchers created the 3-D-printed hydrogel matrix to house the nanoparticles, forming a device that mimics the function of the liver by sensing, attracting and capturing toxins routed from the blood.
“These nano-enabled hydrogels can maintain and stabilize the functions of the encapsulated nanoparticles and prevent aggregation,” Chen explained. “In addition, the biomimetic hydrogels can be engineered with fully synthetic or naturally derived materials to provide more features, such as non-biofouling, size-dependent permeability, among others.”
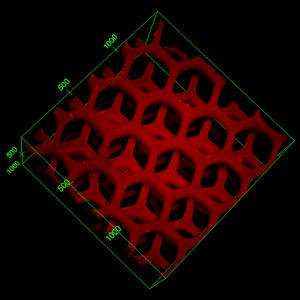 Chen said the overall device mimics the structure of the liver but has larger surface area designed to efficiently attract and trap toxins within the device. In an in vivo study, the device completely neutralized pore-forming toxins.
Chen said the overall device mimics the structure of the liver but has larger surface area designed to efficiently attract and trap toxins within the device. In an in vivo study, the device completely neutralized pore-forming toxins.
“Compared to conventional hemodialysis treatment using semi-permeable dialysis membranes, which is limited to water-soluble small toxins with low volume of distribution and based on passive diffusion, our nano-particle can actively attract and capture the toxins,” Chen explained. “Furthermore, the captured toxins will stay within the hydrogel instead of circulating in the blood stream in the form of toxin-nanoparticle complex, which could be a potential shortcoming for other nanoparticle based technologies.”
“One unique feature of the device is that it turns red when the toxins are captured,” added co-first author, Xin Qu, a postdoctoral researcher working in Chen’s laboratory.
Chen reported that linking the nanoparticles to the hydrogel was one of the key challenges the team faced.
“We managed to chemically modify our nanoparticle with an acryloyl group on the surface and utilize photo-polymerization to link the particles with our hydrogel backbones,” he explained. “This modification scheme was proven to secure the nanoparticles in addition to the physical confinement due to the tortuous nature of hydrogel network.”
Chen added that the team now is focusing on system optimization and enhancement.
“We are optimizing our system towards implantable tissue-engineered constructs, which will be self-sustained, can integrate with the host circulation and provide more hepatic function,” he said.
The biofabrication technology Chen developed to make the liver device is called dynamic optical projection stereolithography (DOPsL). It can produce the micro- and nanoscale resolution required to print tissues that mimic nature’s fine-grained details, including blood vessels, which are essential for distributing nutrients and oxygen throughout the body.
The biofabrication technique uses a computer projection system and precisely controlled micromirrors to shine light on a selected area of a solution containing photo-sensitive biopolymers and cells. This photo-induced solidification process forms one layer of solid structure at a time, but does so in a continuous fashion.
Chen’s lab has already demonstrated the ability to print complex 3-D microstructures, such as blood vessels, in seconds out of soft biocompatible hydrogels that contain living cells.
Chen developed the new biofabrication technology under a four year $1.5 million grant from the National Institutes of Health. It represents one goal for Chen, who says he has been working in this area for the past 15 years.
“I have always been interested in advanced technologies for medical uses to help patients,” Chen explained. “Three-dimensional printing was developed for non-medical use at the beginning. But I felt that each disease treatment is patient specific, and 3-D printing is a unique tool to manufacture customer-fit products. Combining these two makes a great sense to me to develop the 3-D bioprinting technologies.

