New Clues Why Older Women Are More Susceptible to Breast Cancer
 The idea that breast cancer becomes more prevalent with age is fairly well established, but the reasons why are still uncertain. Now, scientists from the Lawrence Berkeley National Laboratory have new insights into why older women are more susceptible to breast cancer.
The idea that breast cancer becomes more prevalent with age is fairly well established, but the reasons why are still uncertain. Now, scientists from the Lawrence Berkeley National Laboratory have new insights into why older women are more susceptible to breast cancer.
In a series of experiments that show how cells in the breast react to specific physical stimuli at different ages, a team of researchers led by Mark LaBarge, has shown that as cells age they respond differently to the stimuli applied.
What they found was that in older women the cells responsible for maintaining healthy breast tissue stop responding to their immediate surroundings, including physical cues that should prompt them to suppress nearby tumors. The work sheds light on how aging alters cellular and molecular functions and how these changes contribute to the prevalence of breast cancer in older women—a disease that is most frequently diagnosed among women aged 55 to 64, according to the National Cancer Institute.
“The importance of our work is that we have a system that facilitates a detailed analysis of cellular and molecular level functional consequences of aging in a human epithelia,” LaBarge told Bioscience Technology and Drug Discovery & Development.
The research appeared in the journal Cell Reports. The research team included members from Lawrence Berkeley Lab, University of California-Berkeley and the University of Bergen in Norway.
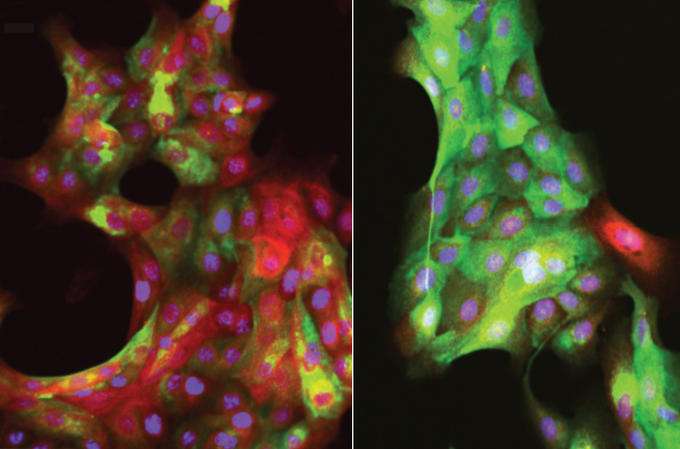
LaBarge’s group studied multipotent progenitors, a type of adult stem cell that is believed to be the origin of many breast cancers. Two years ago, his group found that as women age, multipotent progenitors accumulate in breast epithelial tissue. They didn’t know why these cells increase in numbers, but they believed their cellular microenvironment—the matrix of tissue surrounding the cells—played a role.
To explore this idea, the scientists examined human mammary epithelial cell samples from pre- and post-menopausal women. They wanted to learn how the multipotent progenitors in the tissue samples are affected by changes to their microenvironments.
In tests they placed the tissue on soft polymer surfaces that mimic healthy breast tissue, as well as on progressively stiffer polymer surfaces that mimic the rigidity of a tumor.
They found that multipotent progenitors in tissue from women less than 30 years old are extremely responsive to changes to their immediate surroundings. When tissue from young women was placed on a soft polymer, multipotent progenitors differentiated into milk-producing luminal cells. When the tissue was placed on a stiff polymer, the cells reduced the production of luminal cells and ramped up the production of tumor-fighting myoepithelial cells.
 “We think this is a defense mechanism,” said LaBarge. “The epithelia tissue recognizes that stiffness isn’t good and produces tumor suppressants.”
“We think this is a defense mechanism,” said LaBarge. “The epithelia tissue recognizes that stiffness isn’t good and produces tumor suppressants.”
But this defense wasn’t observed in tissue from women older than 55. Instead of responding to a stiff polymer by upping the production of tumor-suppressing cells, multipotent progenitors from older women produced equal amounts of luminal and tumor-suppressing cells. They also made more of themselves, which is not good. The majority of cancers diagnosed in older women are luminal, and more multipotent progenitors means there are more cells that can become cancerous.
“We found that as women age, multipotent progenitors, the cells responsible for maintaining healthy homeostasis in breast tissue, no longer respond to their microenvironment like they do in younger women,” said LaBarge, who has been involved in this line of research since his doctoral work at Stanford.
“One reason for this is that multipotent progenitors in older tissue do not correctly perceive differentiation cues, such as the mechanical stiffness of their surroundings,” he added.
The scientists traced this failure to a breakdown in a cellular process that converts external mechanical cues into an internal molecular message that tells the cell nucleus what to do. In multipotent progenitors in women older than 55, the molecules that help deliver this message are inefficiently activated, LaBarge’s group found. They believe this breakdown stems from changes in the way women’s genes are activated and silenced as they grow older.
The goal now for LaBarge is to use this information to develop some type of breast cancer prevention strategy.
“We are using our system to look for ways that the loss in function within the progenitor population can be avoided, or possibly reversed, thus retaining or restoring the defensive mechanisms in breast tissue as women age,” he said.
LaBarge’s goal is to find mechanisms that can slow down the aging process of the cells, keeping them more robust and able to detect changes in their surroundings.
“We are working on identifying the mechanisms by which the different lineages age, then on trying to slow down that process,” he explained. “Ideally we will identify compounds that can achieve this safely, so safe that even very low-risk women could benefit from some form of chemoprevention.”
“My ideal would be analogous to taking a daily aspirin to prevent heart disease,” he explained. “I want to find the aspirin-equivalent for breast cancer.”
Breast Research Relies on Unusual Cell Collection
One key tool used by Mark LaBarge and his team in their breast cancer studies is the human mammary epithelial cell (HMEC) Aging Resource. The resource is a large collection of breast tissue samples from healthy women, aged 16 to 91.
Human mammary epithelial cells are one of the few examples of an epithelial tissue that affords relatively good access because of mastectomies and cosmetic reduction surgeries. In both cases, surgical discards provide sample tissue for research.
In addition, LaBarge and his colleagues had access to frozen surgical HMEC tissue specimens acquired by co-author Martha Stampfer some 30 years ago when she began developing HMEC cultures for breast cancer research. Using a unique cell culture system, the LaBarge team was able to generate a large collection of normal HMEC strains derived from primary tissue in women.
“We do not know of another resource in the world like this for studying any type of human tissue,” LaBarge said. “With this resource, we were able to perform functional studies of normal HMECs to observe the cellular changes that occurred with aging.”

